Difference between revisions of "Liquid-liquid extraction - 2014"
Kevin Dunn (talk | contribs) |
Kevin Dunn (talk | contribs) |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{ClassSidebarYouTube | ||
| date = 29 October 2014 | | date = 29 October 2014 | ||
| dates_alt_text = | | dates_alt_text = | ||
| vimeoID1 = | | vimeoID1 = N3CsY6oANhw | ||
| vimeoID2 = | | vimeoID2 = hz1HaKJd5pU | ||
| vimeoID3 = | | vimeoID3 = gX7sKIes0Zo | ||
| vimeoID4 = | | vimeoID4 = v-mNuDk-p2s | ||
| vimeoID5 = | | vimeoID5 = 6rChTv_D5sU | ||
| vimeoID6 = | | vimeoID6 = PkOeNkUbVxw | ||
| vimeoID7 = | | vimeoID7 = | ||
| vimeoID8 = | | vimeoID8 = | ||
| Line 26: | Line 26: | ||
| video_notes2 = | | video_notes2 = | ||
| video_download_link3_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-10B.mp4 | | video_download_link3_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-10B.mp4 | ||
| video_download_link3_MP4_size = M | | video_download_link3_MP4_size = 553 M | ||
| video_notes3 = | | video_notes3 = | ||
| video_download_link4_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-10C.mp4 | | video_download_link4_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-10C.mp4 | ||
| video_download_link4_MP4_size = M | | video_download_link4_MP4_size = 586 M | ||
| video_notes4 = | | video_notes4 = | ||
| video_download_link5_MP4 = | | video_download_link5_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-11A.mp4 | ||
| video_download_link5_MP4_size = M | | video_download_link5_MP4_size = 797 M | ||
| video_notes5 = | | video_notes5 = | ||
| video_download_link6_MP4 = | | video_download_link6_MP4 = http://learnche.mcmaster.ca/media/2014-4M3-Class-11B.mp4 | ||
| video_download_link6_MP4_size = M | | video_download_link6_MP4_size = 357 M | ||
| video_notes6 = | | video_notes6 = | ||
| video_download_link7_MP4 = | | video_download_link7_MP4 = | ||
| Line 52: | Line 52: | ||
* Schweitzer, "Handbook of Separation Techniques for Chemical Engineers", Chapter 1.9, [http://catalogue.mcmaster.ca/catalogue/Record/1156427 McMaster library] | * Schweitzer, "Handbook of Separation Techniques for Chemical Engineers", Chapter 1.9, [http://catalogue.mcmaster.ca/catalogue/Record/1156427 McMaster library] | ||
* Seader, Henley and Roper, "Separation Process Principles", Chapter 8 in 2nd and 3rd edition [http://catalogue.mcmaster.ca/catalogue/Record/1270236 McMaster Libraries] (reserve) | * Seader, Henley and Roper, "Separation Process Principles", Chapter 8 in 2nd and 3rd edition [http://catalogue.mcmaster.ca/catalogue/Record/1270236 McMaster Libraries] (reserve) | ||
== Resources == | |||
''Scroll down, if necessary, to see the resources.'' | |||
{| class="wikitable" style="text-align: center;" | {| class="wikitable" style="text-align: center;" | ||
| Line 71: | Line 74: | ||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-09B.mp3 Audio] | | [http://learnche.mcmaster.ca/media/2014-4M3-Class-09B.mp3 Audio] | ||
|align="left" colspan="1"| | |align="left" colspan="1"| | ||
A comprehensive article on [http://learnche.mcmaster.ca/media/mcmaster/Bailes-Hanson-Hughes--Liquid-liquid-extraction.pdf | A comprehensive article on [http://learnche.mcmaster.ca/media/mcmaster/Bailes-Hanson-Hughes--Liquid-liquid-extraction.pdf liquid-liquid extraction] which describes the various units available. | ||
|- | |- | ||
| 04 November | | 04 November | ||
| Line 83: | Line 86: | ||
|align="left" colspan="1"| | |align="left" colspan="1"| | ||
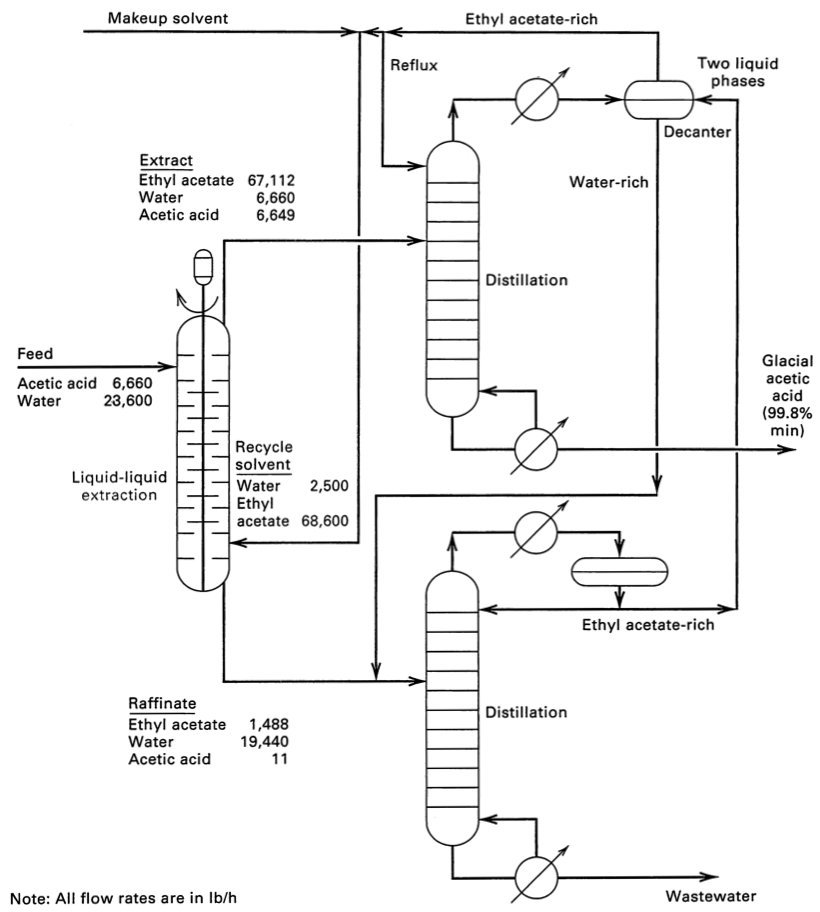
* The flowsheet for separating acetic acid from water using ethyl acetate solvent. This flowsheet has the mass flow rates, to help contrast it to distillation. | * The flowsheet for separating acetic acid from water using ethyl acetate solvent. This flowsheet has the mass flow rates, to help contrast it to distillation. | ||
:[[Image:Acetic-acid-water-ethyl-acetate-flowsheet-Seader-3ed-p300.jpg|300px]] <span style="color:#8822AA">''click image to enlarge''</span> [reference: Seader ''et al.'', p300] | :[[Image:Acetic-acid-water-ethyl-acetate-flowsheet-Seader-3ed-p300.jpg|300px]] <span style="color:#8822AA">''click image to enlarge''</span> | ||
[reference: Seader ''et al.'', p300] | |||
|- | |- | ||
| 05 November | | 05 November | ||
| 10B | | 10B | ||
| align="left" colspan="1"| | | align="left" colspan="1"| | ||
Liquid liquid extraction | Liquid liquid extraction example calculations | ||
| align="left" colspan="1"| | | align="left" colspan="1"| | ||
[[Media:2014-4M3-Liquid-Liquid-Extraction.pdf|Slides]] | [[Media:2014-4M3-Liquid-Liquid-Extraction.pdf|Slides]] | ||
| | | [http://learnche.mcmaster.ca/media/2014-4M3-Class-10B.mp4 Video] | ||
| | | [http://learnche.mcmaster.ca/media/2014-4M3-Class-10B.mp3 Audio] | ||
|align="left" colspan="1"| | |align="left" colspan="1"| | ||
* The [[Media:Seader-ternary-example-base.pdf|ternary diagram that will be used in today's class]]. Download and print it out for practice problems. | * The [[Media:Seader-ternary-example-base.pdf|ternary diagram that will be used in today's class]]. Download and print it out for practice problems. | ||
* Here's [http://webpages.sdsmt.edu/~ddixon/CBE417_Lec_23_LLE_Draft_3-print.pdf another instructor's slides] on the same topic - sometimes a different point of view helps to understand. | |||
|- | |||
| 07 November | |||
| 10C | |||
| align="left" colspan="1"| | |||
Liquid liquid extraction example calculations | |||
| align="left" colspan="1"| | |||
[[Media:2014-4M3-Liquid-Liquid-Extraction.pdf|Slides]] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-10C.mp4 Video] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-10C.mp3 Audio] | |||
|align="left" colspan="1"| | |||
Web links shown in the class: | |||
* [http://modularprocess.com/liquid-extraction/extraction-column-types/rdc/ Rotating disc column] | |||
* [http://modularprocess.com/liquid-extraction/extraction-column-types/karr-columns/ Karr column] | |||
* [http://modularprocess.com/liquid-extraction/extraction-column-types/scheibel-columns/ Scheibel column] | |||
* [http://modularprocess.com/liquid-extraction/extraction-column-types/other-columns/ Pulsed and packed columns] | |||
|- | |||
| 11 November | |||
| 11A | |||
| align="left" colspan="1"| | |||
Liquid liquid extraction example calculations | |||
| align="left" colspan="1"| | |||
[[Media:2014-4M3-Liquid-Liquid-Extraction.pdf|Slides]] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-11A.mp4 Video] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-11A.mp3 Audio] | |||
|align="left" colspan="1"| | |||
These readings seem old, but they are still relevant. For example, the same principles are used in modern bioseparations. | |||
# A [http://learnche.mcmaster.ca/media/mcmaster/Hanson--Solvent-extraction.pdf reading on solvent extraction principles] | |||
# A general article on [http://learnche.mcmaster.ca/media/mcmaster/Reissinger-Schroter--Liquid-liquid-extractors.pdf liquid liquid extractors]. | |||
# An interesting [http://learnche.mcmaster.ca/media/mcmaster/Moore--Using-principles-of-inherent-safety-in-design-of-hydrometallurgical-solvent-extraction-plants.pdf reading on safety in liquid-liquid extraction plants]: a further reason for counter-current operations to minimize solvent use. | |||
|- | |||
| 12 November | |||
| 11B | |||
| align="left" colspan="1"| | |||
Liquid liquid extraction example calculations | |||
| align="left" colspan="1"| | |||
[[Media:2014-4M3-Liquid-Liquid-Extraction.pdf|Slides]] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-11B.mp4 Video] | |||
| [http://learnche.mcmaster.ca/media/2014-4M3-Class-11B.mp3 Audio] | |||
|align="left" colspan="1"| | |||
|} | |} | ||
== Exercise == | |||
A stream of acetic acid and water (also called diluent) is being fed in a counter current manner at 1000 kg/hour, in order to extract the acetic acid. The feed composition is 30 wt% acetic acid, and 70 wt% water. | |||
The solvent is 99% pure IPE (isopropyl ether), and contains 1% acetic acid, at an inlet flow of 2500 kg/hour. | |||
We desire the exiting raffinate stream to contain 5 wt% acetic acid. | |||
# Find the number of equilibrium stages to achieve this separation (show all calculations). | |||
# Calculate the exiting raffinate flow, and the exiting extract flow rate. | |||
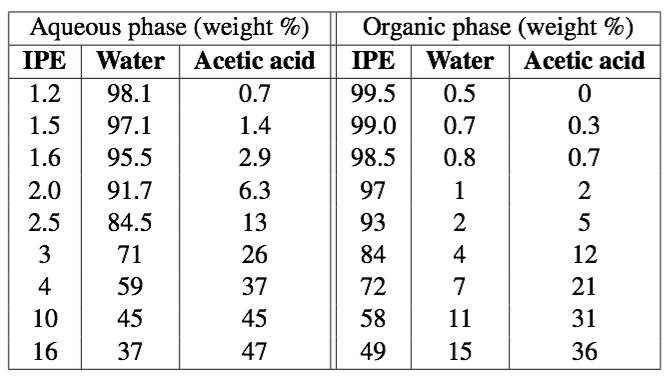
Unfortunately, we don't have the equilibrium data, however, various samples of the 3 species were made, mixed, and when they came to equilibrium they were found to have the following compositions (each row gives the aqueous and organic phase compositions): | |||
[[Image:Adsorption-example-from-VLE-raw-data.png|500px]] | |||
Feel free to download and use [http://upload.wikimedia.org/wikipedia/commons/8/81/Ternary_plot_1.png this empty ternary diagram]. | |||
See [http://www.youtube.com/watch?v=N7MIH0_ELO0 this YouTube video] for the full solution.. | |||
<!-- | <!-- | ||
'''← these are new''' | '''← these are new''' | ||
--> | --> | ||
Latest revision as of 08:57, 6 January 2017
| Class date(s): | 29 October 2014 | ||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
References
Please use these references to read ahead, or for extra background reading on liquid-liquid extraction. In alphabetical order:
- Ghosh, R. "Principles of Bioseparations Engineering", Chapter 7, McMaster (reserve)
- Geankoplis, C.J. "Transport Processes and Separation Process Principles", Chapter 12 in 3rd and 4th edition, McMaster Libraries (reserve)
- Perry's Chemical Engineers' Handbook, Chapter 15, Direct link (McMaster subscription)
- Richardson and Harker, "Chemical Engineering, Volume 2", 5th edition, Chapter 13 ebook
- Schweitzer, "Handbook of Separation Techniques for Chemical Engineers", Chapter 1.9, McMaster library
- Seader, Henley and Roper, "Separation Process Principles", Chapter 8 in 2nd and 3rd edition McMaster Libraries (reserve)
Resources
Scroll down, if necessary, to see the resources.
| Date | Class number | Topic | Slides for class | Video and audio files | References and Notes | |
|---|---|---|---|---|---|---|
| 29 October | 09B |
Liquid liquid extraction overview |
Video | Audio |
A comprehensive article on liquid-liquid extraction which describes the various units available. | |
| 04 November | 10A |
Liquid liquid extraction theory and calculations |
Video | Audio |
[reference: Seader et al., p300] | |
| 05 November | 10B |
Liquid liquid extraction example calculations |
Video | Audio |
| |
| 07 November | 10C |
Liquid liquid extraction example calculations |
Video | Audio |
Web links shown in the class: | |
| 11 November | 11A |
Liquid liquid extraction example calculations |
Video | Audio |
These readings seem old, but they are still relevant. For example, the same principles are used in modern bioseparations.
| |
| 12 November | 11B |
Liquid liquid extraction example calculations |
Video | Audio | ||
Exercise
A stream of acetic acid and water (also called diluent) is being fed in a counter current manner at 1000 kg/hour, in order to extract the acetic acid. The feed composition is 30 wt% acetic acid, and 70 wt% water.
The solvent is 99% pure IPE (isopropyl ether), and contains 1% acetic acid, at an inlet flow of 2500 kg/hour.
We desire the exiting raffinate stream to contain 5 wt% acetic acid.
- Find the number of equilibrium stages to achieve this separation (show all calculations).
- Calculate the exiting raffinate flow, and the exiting extract flow rate.
Unfortunately, we don't have the equilibrium data, however, various samples of the 3 species were made, mixed, and when they came to equilibrium they were found to have the following compositions (each row gives the aqueous and organic phase compositions):
Feel free to download and use this empty ternary diagram.
See this YouTube video for the full solution..