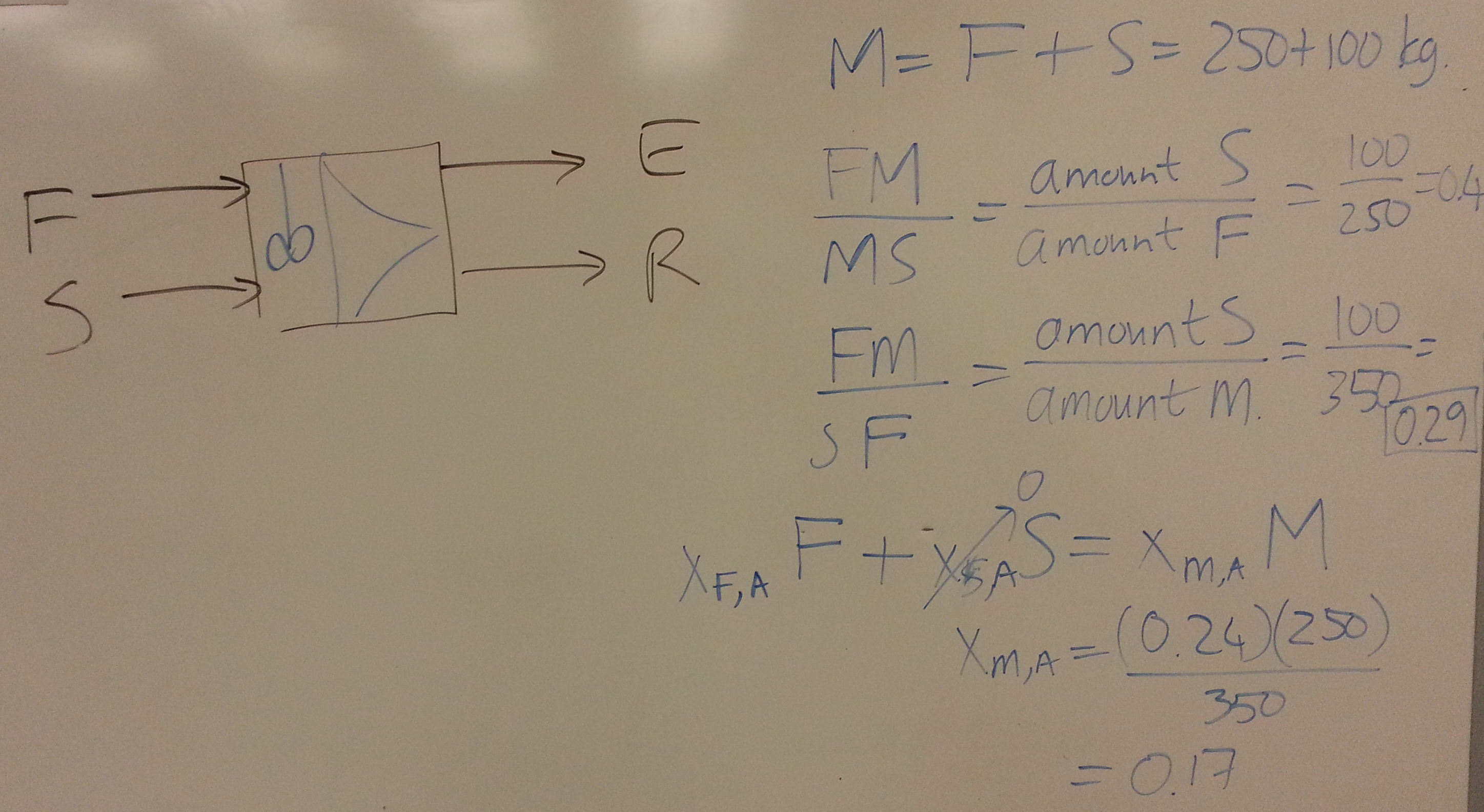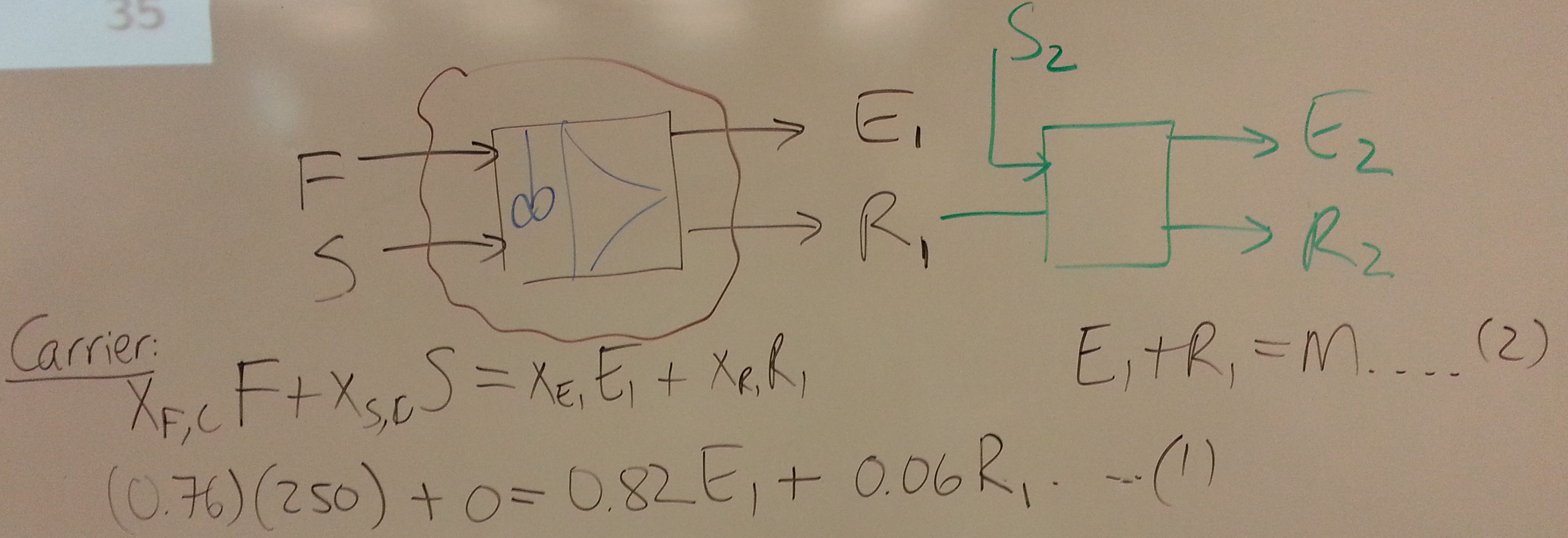| Class date(s):
|
23 to 26 October 2012
|
|
|
|
|
| Download video: Link [102 M]
|
|
|
|
|
|
|
|
|
| Download video: Link [123 M]
|
|
|
|
|
|
|
|
|
| Download video: Link [127 M]
|
|
|
|
|
|
|
|
|
| Download video: Link [119 M]
|
|
|
|
|
|
|
|
|
| Download video: Link [119 M]
|
|
|
|
|
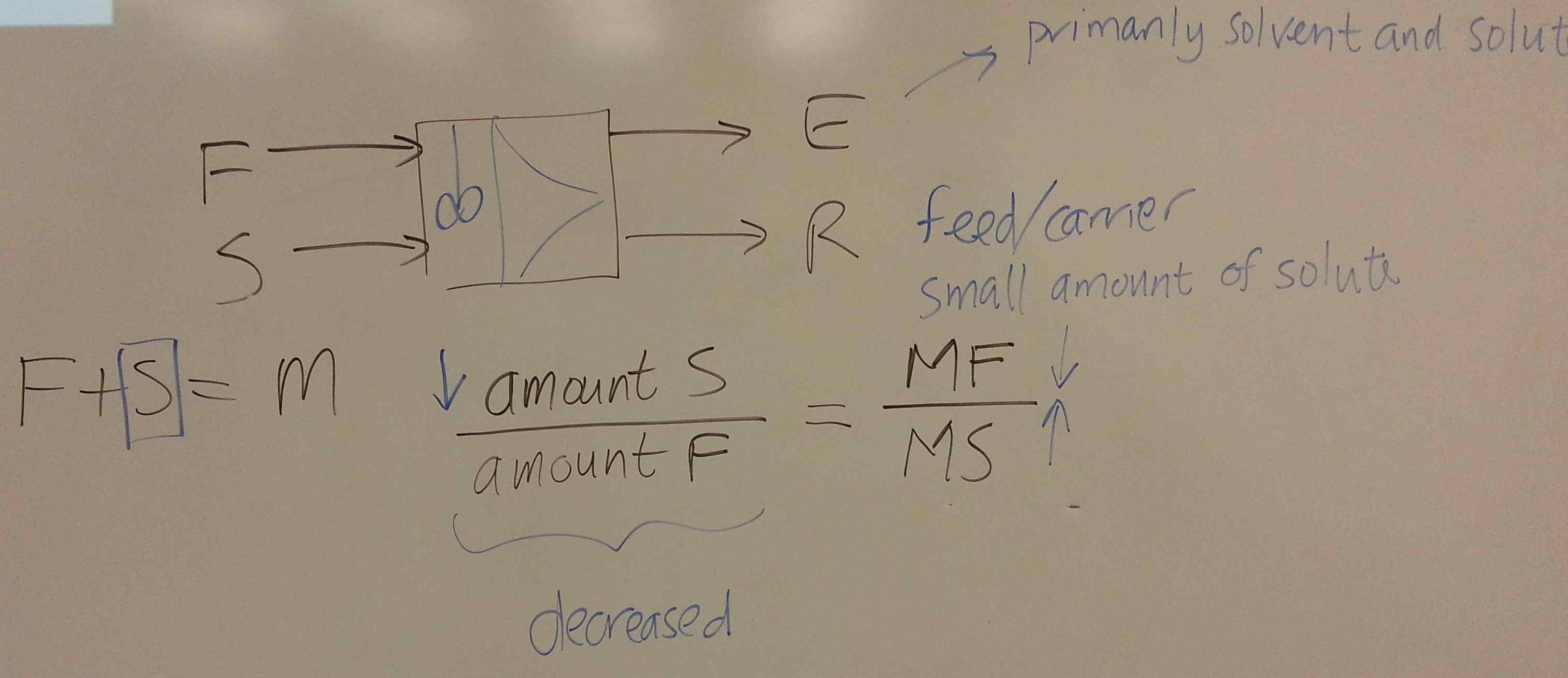
We start this section by looking at liquid-liquid extraction.
References
Please use these references to read ahead, or for extra background reading on liquid-liquid extraction. In alphabetical order:
- Ghosh, R. "Principles of Bioseparations Engineering", Chapter 7, McMaster (reserve)
- Geankoplis, C.J. "Transport Processes and Separation Process Principles", Chapter 12 in 3rd and 4th edition, McMaster Libraries (reserve)
- Perry's Chemical Engineers' Handbook, Chapter 15, Direct link (McMaster subscription)
- Richardson and Harker, "Chemical Engineering, Volume 2", 5th edition, Chapter 13 ebook
- Schweitzer, "Handbook of Separation Techniques for Chemical Engineers", Chapter 1.9, McMaster library
- Seader, Henley and Roper, "Separation Process Principles", Chapter 8 in 2nd and 3rd edition McMaster Libraries (reserve)
Interesting applications / Enrichment materials
- An article on liquid-liquid extraction which describes the various units available.
- The flowsheet for separating acetic acid from water using ethyl acetate solvent. This flowsheet has the mass flow rates, to help contrast it to distillation.
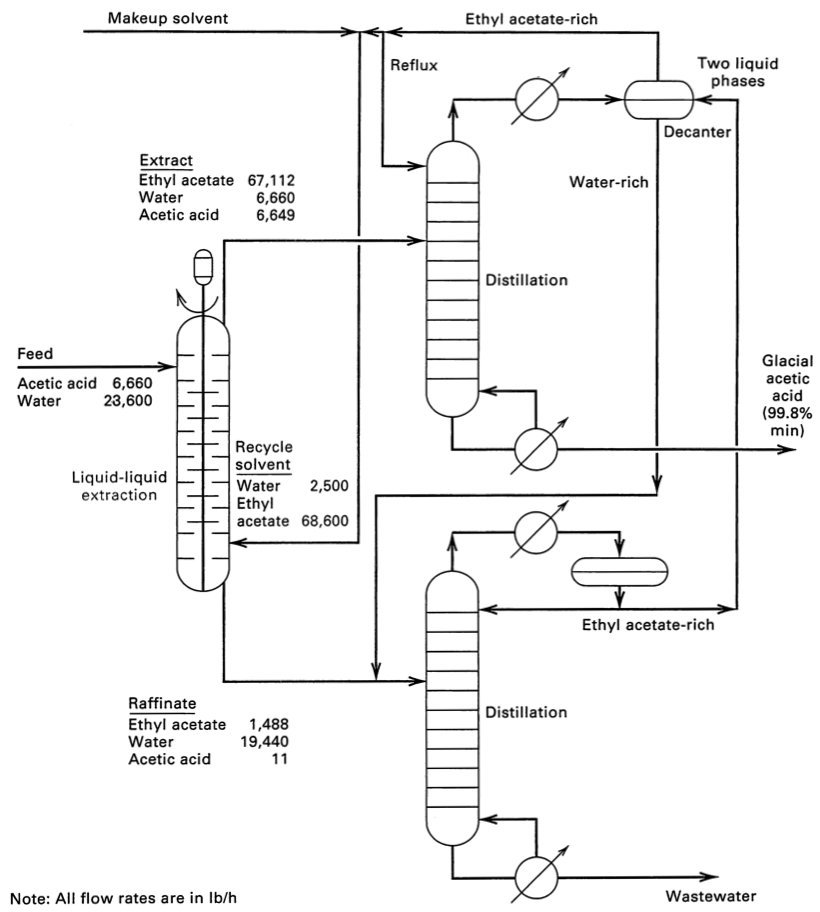
- click to enlarge
Week 8
23 Oct 2012 (08A)
25 Oct 2012 (08B)
26 Oct 2012 (08C)
 click to enlarge
click to enlarge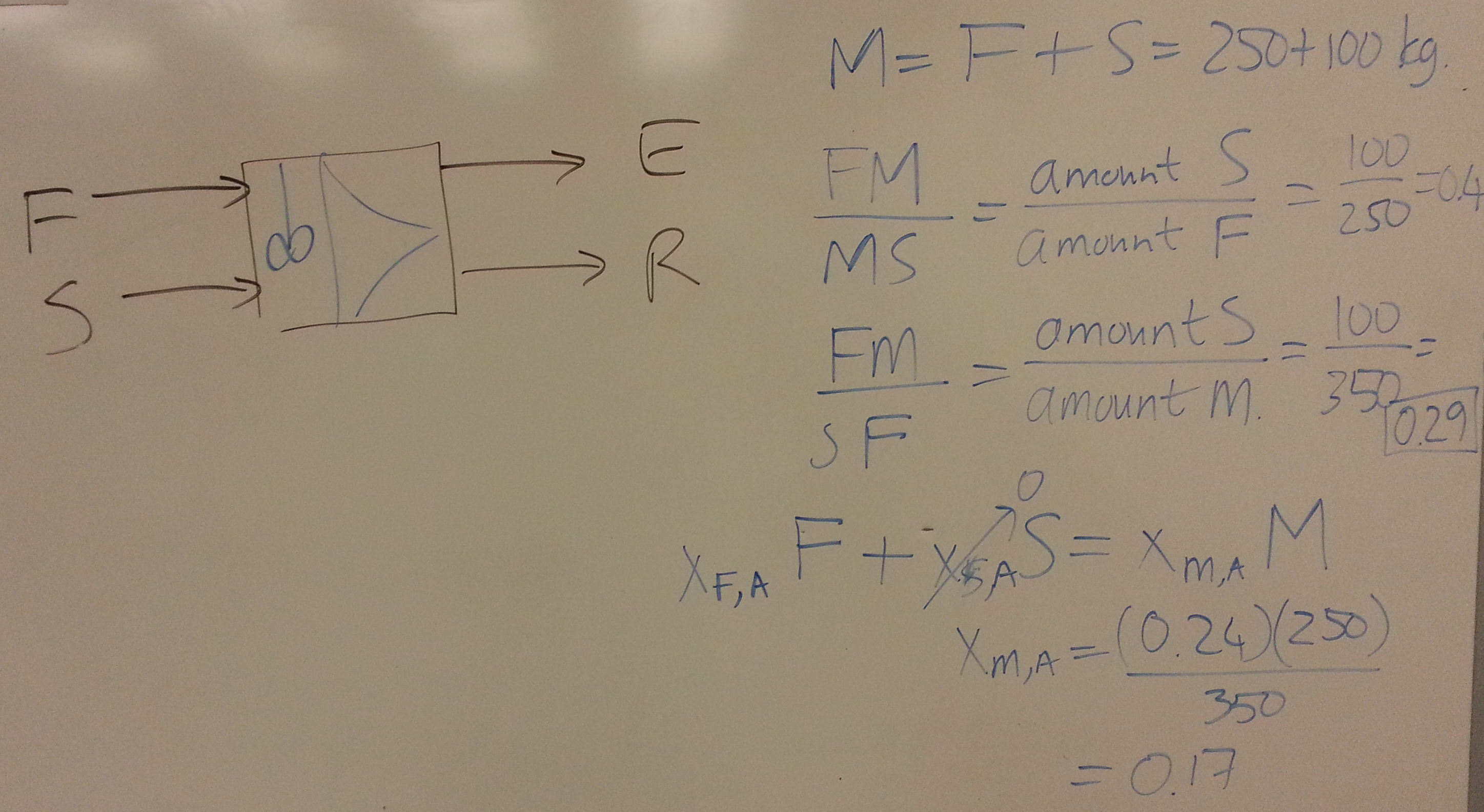 click to enlarge
click to enlarge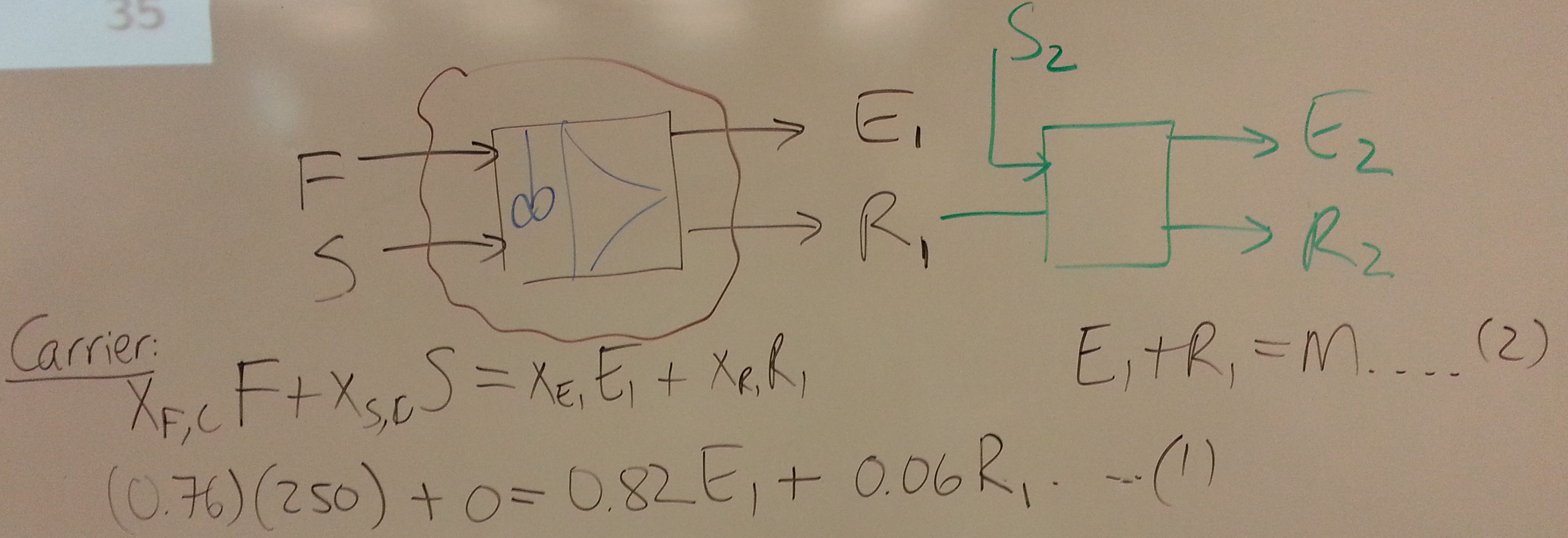 click to enlarge
click to enlarge
Week 9
30 Oct 2012 (09A)
We will consider single and multiple co-current extraction.
 click to enlarge
click to enlarge
31 Oct 2012 (09B)
We should be able to wrap up the section by studying counter-current liquid-liquid extraction.
- Slides for class Please print slides 48 to 54 on large, single pages, to assist your learning.
- Audio and video recording of the class.
- Photo of the board during class
 click to enlarge
click to enlarge
01 Nov 2012 (09C)
- Slides for class (slide 58)
- There will be no formal teaching in class today. You can ask questions about assignment 4, and there will be a tutorial question from assignment 5.
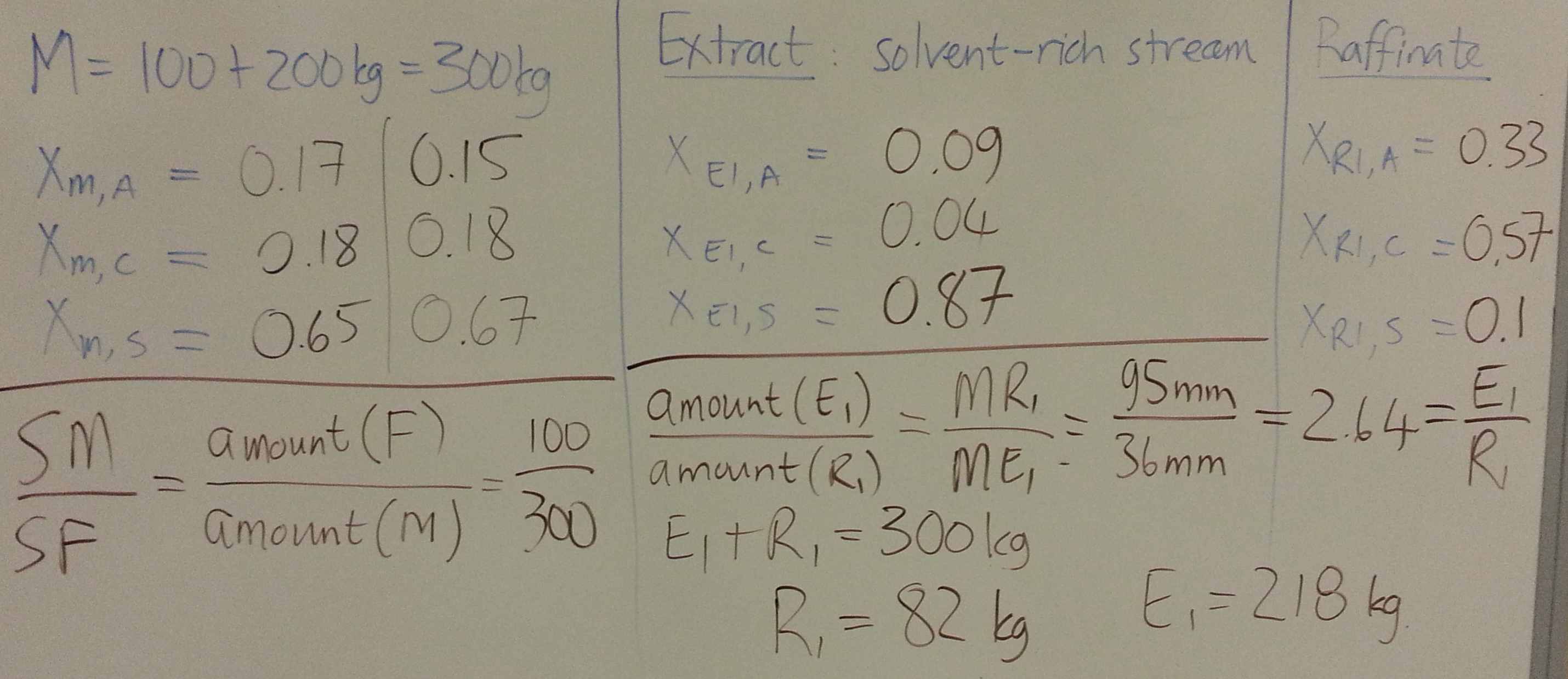
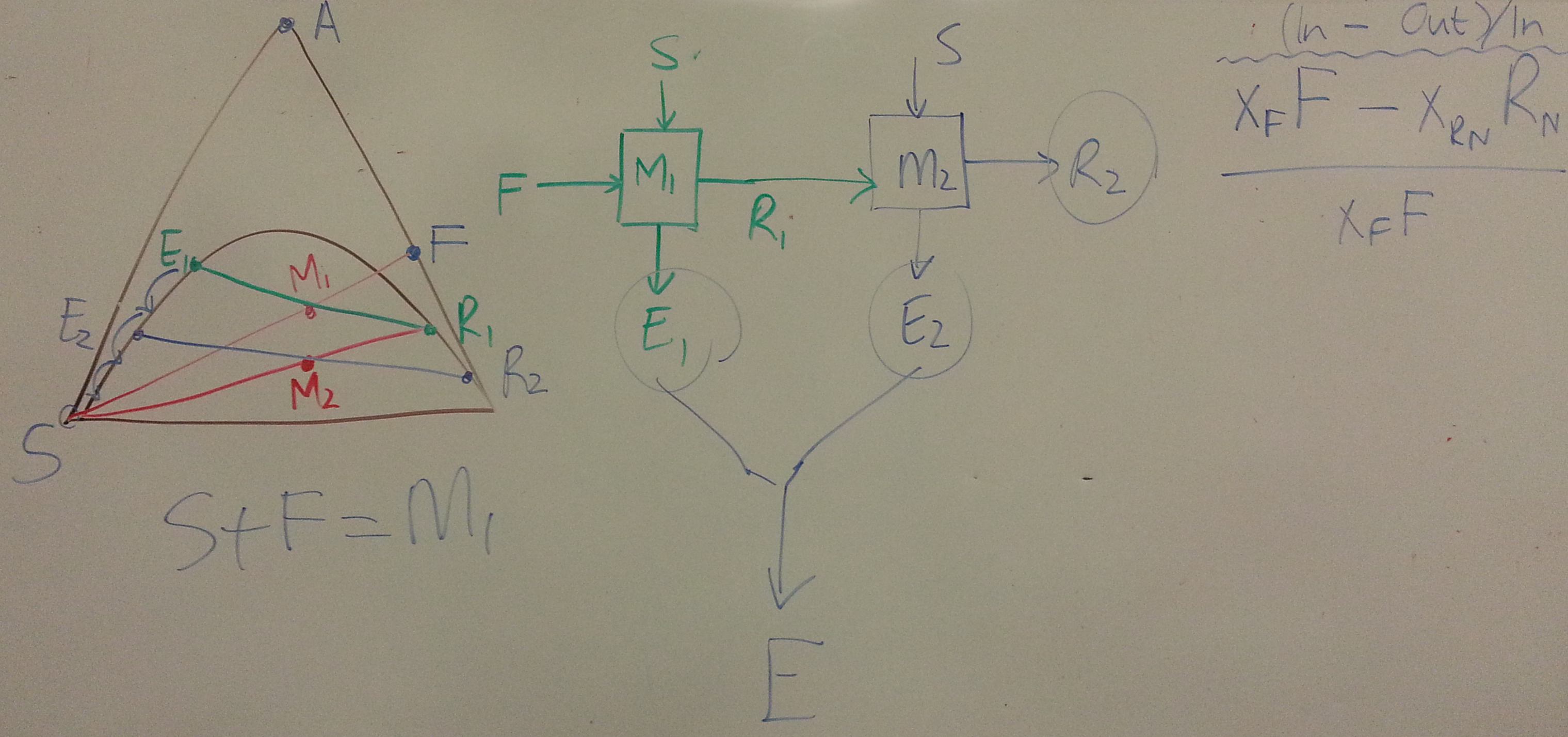
- Consider a system for which you have been given the ternary diagram (see slide 59 and 60). \(A\) = solute, \(S\) = solvent, \(C\) = carrier. The feed, \(F\) enters at 112 kg/hr with composition of 25 wt% solute and 75 wt% carrier.
- Calculate the flow and composition of the extract and raffinate from:
- 1st co-current stage, using a pure solvent flow of 50 kg/hr.
- 2nd co-current stage, with an additional solvent flow of 50 kg/hr.
- For the overall 2-stage system, find the:
- overall recovery
- overall concentration of combined extract streams
- The next objective is to have a counter-current system so the raffinate leaving in the \(N^\text{th}\) stage, \(R_N\) has \(y_{R_N} = 0.025\)
- What is the maximum allowable solvent flow?
- Explain whether it's possible to achieve an extract stream of \(y_{E_1} = 0.21\)?
- Show the construction on the ternary diagram for the number of equilibrium stages to achieve \(y_{R_N} = 0.025\), given a solvent flow of 60 kg/hr.
- Plot on the same axes the concentrations in the extract and raffinate streams.