Difference between revisions of "Liquid-liquid extraction - 2014"
Kevin Dunn (talk | contribs) |
Kevin Dunn (talk | contribs) |
||
| Line 142: | Line 142: | ||
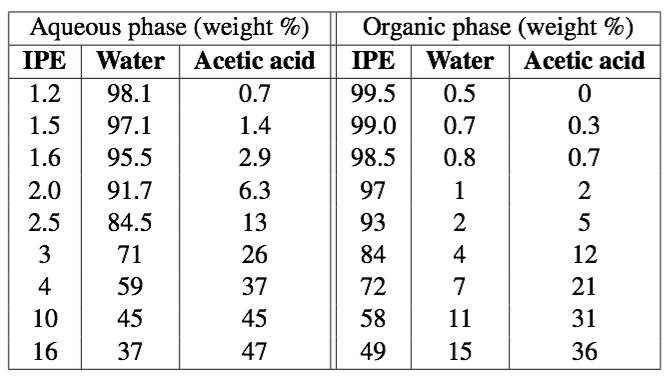
Unfortunately, we don't have the equilibrium data, however, various samples of the 3 species were made, mixed, and when they came to equilibrium they were found to have the following compositions (each row gives the aqueous and organic phase compositions): | Unfortunately, we don't have the equilibrium data, however, various samples of the 3 species were made, mixed, and when they came to equilibrium they were found to have the following compositions (each row gives the aqueous and organic phase compositions): | ||
[[Image:Adsorption-example-from-VLE-raw-data.png| | [[Image:Adsorption-example-from-VLE-raw-data.png|500px]] | ||
Feel free to download and use [http://upload.wikimedia.org/wikipedia/commons/8/81/Ternary_plot_1.png this empty ternary diagram]. | Feel free to download and use [http://upload.wikimedia.org/wikipedia/commons/8/81/Ternary_plot_1.png this empty ternary diagram]. | ||
Revision as of 19:09, 12 November 2014
| Class date(s): | 29 October 2014 | ||||
| |||||
| |||||
| |||||
| |||||
|
| |||||
References
Please use these references to read ahead, or for extra background reading on liquid-liquid extraction. In alphabetical order:
- Ghosh, R. "Principles of Bioseparations Engineering", Chapter 7, McMaster (reserve)
- Geankoplis, C.J. "Transport Processes and Separation Process Principles", Chapter 12 in 3rd and 4th edition, McMaster Libraries (reserve)
- Perry's Chemical Engineers' Handbook, Chapter 15, Direct link (McMaster subscription)
- Richardson and Harker, "Chemical Engineering, Volume 2", 5th edition, Chapter 13 ebook
- Schweitzer, "Handbook of Separation Techniques for Chemical Engineers", Chapter 1.9, McMaster library
- Seader, Henley and Roper, "Separation Process Principles", Chapter 8 in 2nd and 3rd edition McMaster Libraries (reserve)
| Date | Class number | Topic | Slides for class | Video and audio files | References and Notes | |
|---|---|---|---|---|---|---|
| 29 October | 09B |
Liquid liquid extraction overview |
Video | Audio |
A comprehensive article on liquid-liquid extraction which describes the various units available. | |
| 04 November | 10A |
Liquid liquid extraction theory and calculations |
Video | Audio |
[reference: Seader et al., p300] | |
| 05 November | 10B |
Liquid liquid extraction example calculations |
Video | Audio |
| |
| 07 November | 10C |
Liquid liquid extraction example calculations |
Video | Audio |
Web links shown in the class: | |
| 11 November | 11A |
Liquid liquid extraction example calculations |
Video | Audio |
These readings seem old, but they are still relevant. For example, the same principles are used in modern bioseparations.
| |
Exercise
A stream of acetic acid and water (also called diluent) is being fed in a counter current manner at 1000 kg/hour, in order to extract the acetic acid. The feed composition is 30 wt% acetic acid, and 70 wt% water.
The solvent is 99% pure IPE (isopropyl ether), and contains 1% acetic acid, at an inlet flow of 2500 kg/hour.
We desire the exiting raffinate stream to contain 5 wt% acetic acid.
- Find the number of equilibrium stages to achieve this separation (show all calculations).
- Calculate the exiting raffinate flow, and the exiting extract flow rate.
Unfortunately, we don't have the equilibrium data, however, various samples of the 3 species were made, mixed, and when they came to equilibrium they were found to have the following compositions (each row gives the aqueous and organic phase compositions):
Feel free to download and use this empty ternary diagram.
See this YouTube video for the full solution..